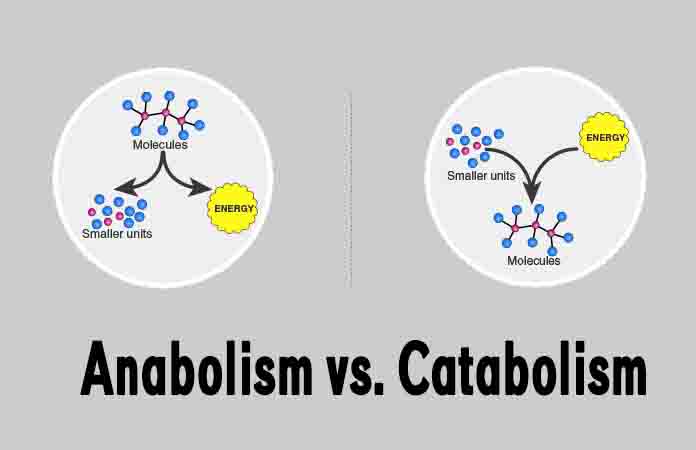
Anabolism and catabolism are the two types of biochemical reactions that make up the metabolism. Anabolic reactions involve the building of larger, complex molecules from smaller, simpler ones, and require an input of energy.
Catabolic reactions are the opposite of anabolic reactions, and break the chemical bonds in larger, more complex molecules. This process releases energy while breaking larger molecules down into their smaller components.
Requires energyReleases energyBuilds larger, complex molecules from smaller, simpler onesBreaks down large, complex molecules into smaller, simpler onesForms chemical bonds between moleculesBreaks chemical bonds within molecules
What is Anabolism?
Anabolic processes are building reactions. These processes use small, simple molecules to create larger, more complex molecules, and require an input of energy to do so. For example, single amino acids may be used to assemble large, complex proteins. Because anabolism involves the synthesis of new biological molecules, it is also known as biosynthesis.
The products of anabolism are often used as structural materials for the building of new cells. Therefore, anabolism is the driving force behind the physical growth of organisms.
Examples of Anabolic Reactions
Photosynthesis
One example of an anabolic reaction is photosynthesis. This is series of biochemical reactions that takes place in the chloroplasts of plants and involves the synthesis of glucose from carbon dioxide gas and water molecules.
Like all anabolic reactions, photosynthesis requires an input of energy and is powered by light energy from the sun.
Glycogen synthesis (AKA glycogenesis) is another example of anabolism. During glycogen synthesis, glucose molecules are assembled into long chains of glycogen, which are used to store energy in the liver and muscles.
What is Catabolism?
Catabolism is the opposite of anabolism. Catabolic processes break large, biological molecules down into smaller, simpler molecules. These reactions involve the breaking of chemical bonds, which is accompanied by a release of energy.
Around 40% of the energy released is used to synthesize ATP molecules (the energy currency of cells). The remaining 60% is released as thermal energy and is absorbed by the body tissues and fluids.
Examples of Catabolic Reactions
Cellular Respiration
Cellular respiration is a type of catabolic reaction that takes place inside every living cell. This process involves the breaking down of glucose molecules to release energy, which is then used to power all other cellular processes.
Respiration may take place in the presence of oxygen (aerobic respiration) or its absence (anaerobic respiration).
Digestion of Food
Another vital type of catabolism is the digestion of food. Digestion involves a series of catabolic reactions that break large food molecules down into smaller, simpler molecules.
For example, proteins are broken down into amino acids; complex carbohydrates are broken down into simple sugars; lipids are broken down into fatty acids and glycerol.